JHM Biopharma adopts synthetic biology technology and leverages its proprietary platform to simulate the production process of natural collagen in the human body. It has independently developed recombinant human collagen with a full-length triple helix structure, which has the same hydroxyproline content and advanced structure as natural human collagen. It has outstanding advantages such as strong stability and no immunogenicity. Compared with recombinant humanized collagen, it demonstrates complete biological activity, high mechanical strength, and superior support performance in vivo applications.
Compared with animal derived collagen, it overcomes limitations such as restricted sourcing and the potential risks of disease and viral transmission associated with animal sources. Consequently, it serves as a fully viable substitute for animal-derived collagen in both medical aesthetics and serious medical applications.
JHM Biopharma possesses core technologies and independent intellectual property rights encompassing the entire production process, from fermentation and separation/purification to final product preparation. It has successfully broken through the large-scale fermentation and purification technology of recombinant human collagen, achieved stable expression under 20 tons fermentation system, and successfully completed the industrial production process development of related products.
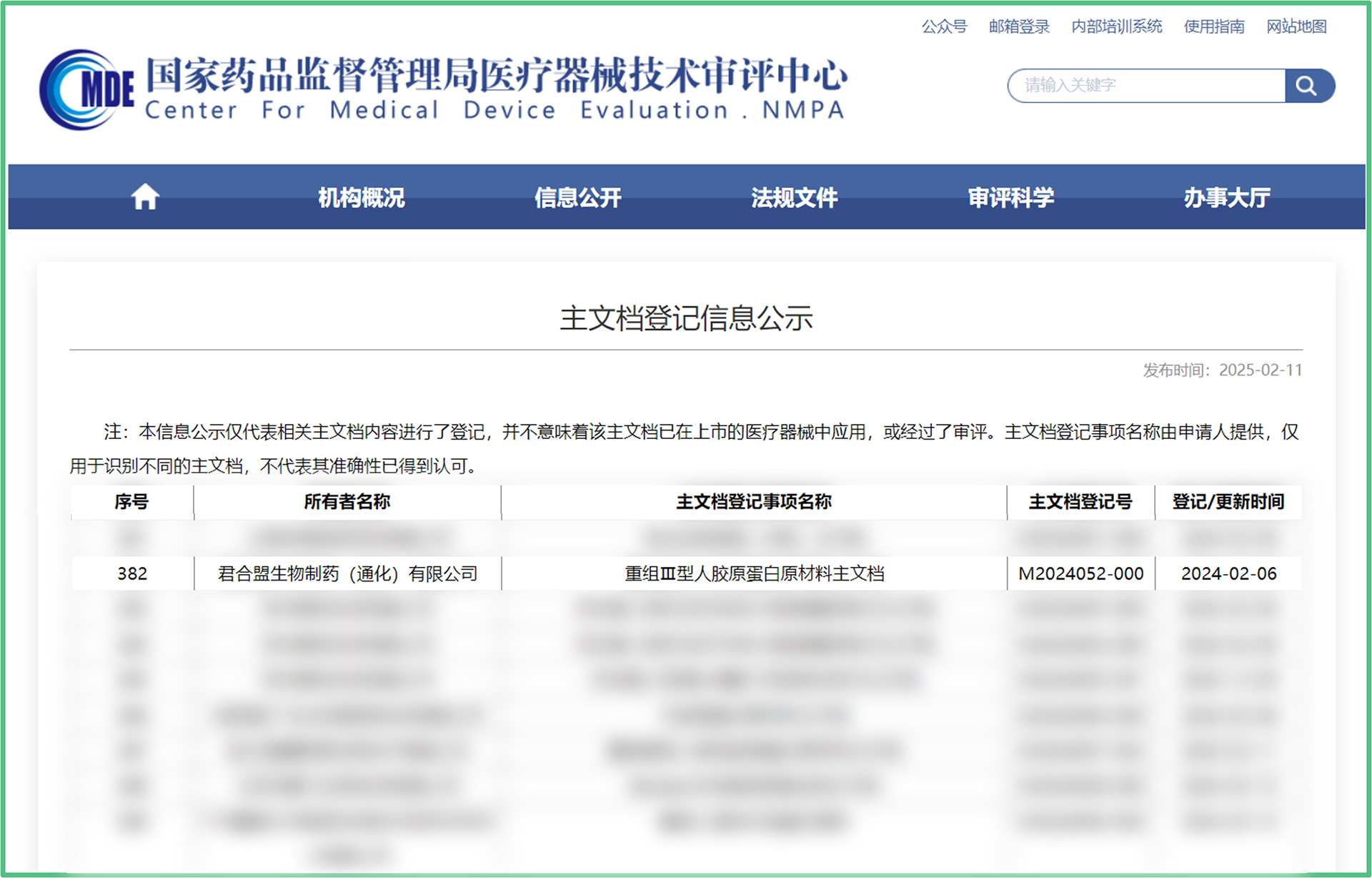
At present, the recombinant type III human collagen protein has completed industrial production and registration of the main document for medical device raw materials (main document registration number: M2024052-000).
More developments in recombinant human collagen, including recombinant type I and type II human collagen, industrial production has been completed, and registration of medical device raw material master documents is currently underway.
JHM Biopharma adopts synthetic biology technology and leverages its proprietary platform to simulate the production process of natural collagen in the human body. It has independently developed recombinant human collagen with a full-length triple helix structure, which has the same hydroxyproline content and advanced structure as natural human collagen. It has outstanding advantages such as strong stability and no immunogenicity. Compared with recombinant humanized collagen, it demonstrates complete biological activity, high mechanical strength, and superior support performance in vivo applications.
Compared with animal derived collagen, it overcomes limitations such as restricted sourcing and the potential risks of disease and viral transmission associated with animal sources. Consequently, it serves as a fully viable substitute for animal-derived collagen in both medical aesthetics and serious medical applications.
JHM Biopharma possesses core technologies and independent intellectual property rights encompassing the entire production process, from fermentation and separation/purification to final product preparation. It has successfully broken through the large-scale fermentation and purification technology of recombinant human collagen, achieved stable expression under 20 tons fermentation system, and successfully completed the industrial production process development of related products.
At present, the recombinant type III human collagen protein has completed industrial production and registration of the main document for medical device raw materials (main document registration number: M2024052-000).
More developments in recombinant human collagen, including recombinant type I and type II human collagen, industrial production has been completed, and registration of medical device raw material master documents is currently underway.